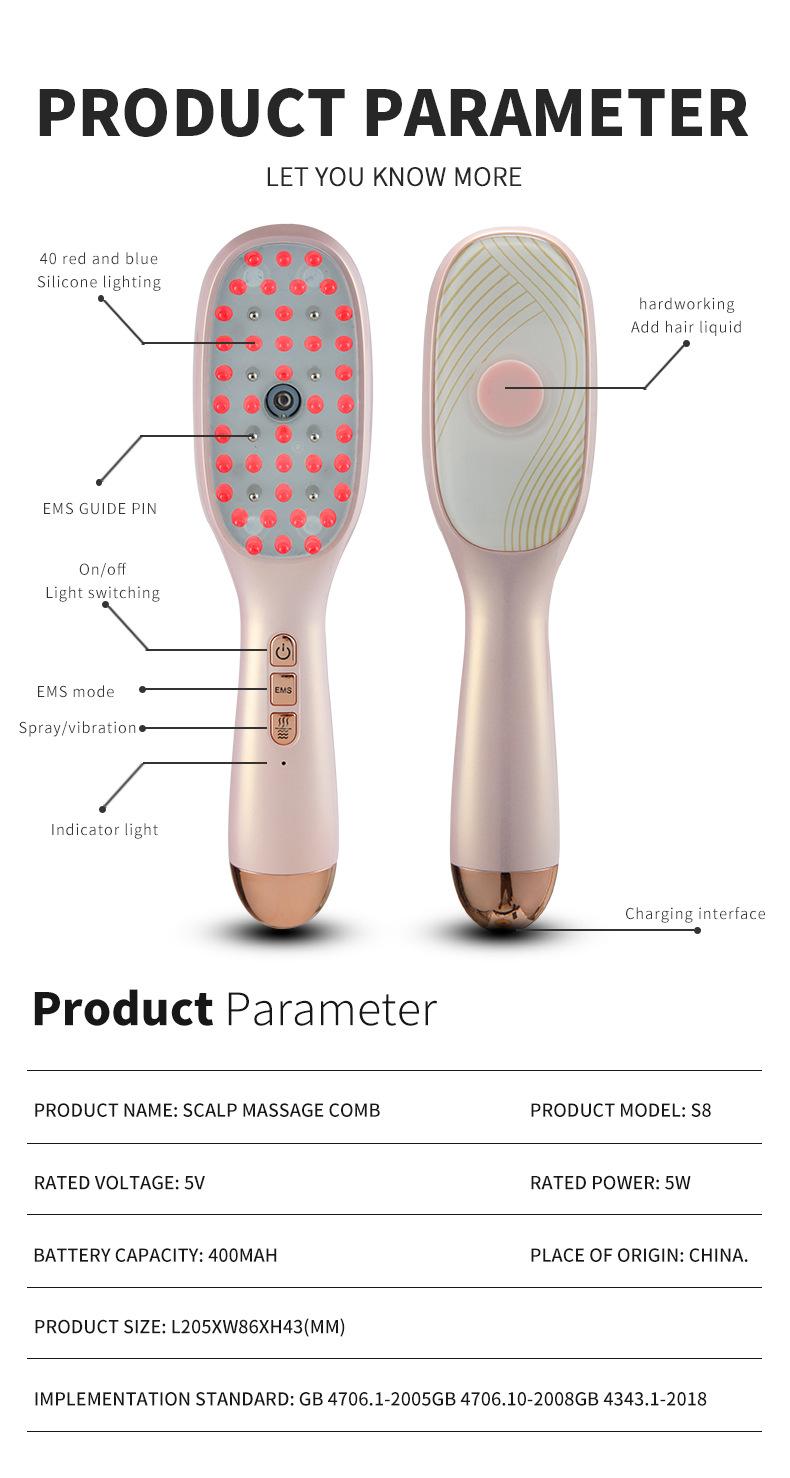
Classified as a medical device ( Electric Scalp Massaging Brush with oil dispencer )
My product with the ASIN B0F1 BDKJS ( Electric Scalp Massaging Brush for Hair Growth, with Oil Applicator for Hair, Portable Smart Scalp Hair Brush, Vibration Comb for Fatigue Relief) Got flagged as a medical device . After speaking with several account health and seller support specialists without any success I must provide the following :
What actions do I need to take?
To reactivate your ASINs, send the following documentation and information:
-- Photos of all the sides of the product packaging.
-- Photos of the instructions included with the product.
-- The 510(k) number issued by the United States Food and Drug Administration (FDA) for this product.
Additionally, if the name of the product provided on the product packaging, instructions for use, or ASIN detail page does not match the device name provided on the 510(k), then also provide the following:
-- The device manufacturer’s FDA establishment registration number or the owner or operator number.
-- The device name as it appears in the manufacturer’s device listing.
-- A purchase order, invoice, or letter, all must be written in English, from the manufacturer confirming that the device was purchased from the manufacturer.
This product is not a medical device . It a comb that vibrates with oil/ water dispenser to moisture the hair . It does not contain medical lasers or is meets the definition of a medical device according to FDA regulation.
Please help ! Other sellers are selling the same products under the ASIN B0DRYD1SRZ and B0DS2L3PBW without any problem...
@Glenn_Amazon @Michelle_Amazon @Cooper_Amazon

Classified as a medical device ( Electric Scalp Massaging Brush with oil dispencer )
My product with the ASIN B0F1 BDKJS ( Electric Scalp Massaging Brush for Hair Growth, with Oil Applicator for Hair, Portable Smart Scalp Hair Brush, Vibration Comb for Fatigue Relief) Got flagged as a medical device . After speaking with several account health and seller support specialists without any success I must provide the following :
What actions do I need to take?
To reactivate your ASINs, send the following documentation and information:
-- Photos of all the sides of the product packaging.
-- Photos of the instructions included with the product.
-- The 510(k) number issued by the United States Food and Drug Administration (FDA) for this product.
Additionally, if the name of the product provided on the product packaging, instructions for use, or ASIN detail page does not match the device name provided on the 510(k), then also provide the following:
-- The device manufacturer’s FDA establishment registration number or the owner or operator number.
-- The device name as it appears in the manufacturer’s device listing.
-- A purchase order, invoice, or letter, all must be written in English, from the manufacturer confirming that the device was purchased from the manufacturer.
This product is not a medical device . It a comb that vibrates with oil/ water dispenser to moisture the hair . It does not contain medical lasers or is meets the definition of a medical device according to FDA regulation.
Please help ! Other sellers are selling the same products under the ASIN B0DRYD1SRZ and B0DS2L3PBW without any problem...
@Glenn_Amazon @Michelle_Amazon @Cooper_Amazon


4 replies
Seller_lmDNUUnN4QXxe
Can a moderator from Amazon help me with this please??
Glenn_Amazon
Hi there @Seller_lmDNUUnN4QXxe,
When the teams are requesting documentation that you provide documentation that demonstrates that it isn't a medical device. You may have been flagged for making some kind of prohibited claim. Once you provide your information to contest the decision and provide your Case ID here I can see what escalation options I can offer. Thank you for your understanding.
-Glenn